Antibody Production
What is an Antibody?
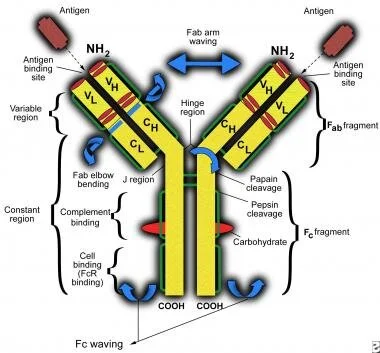
An antibody is a protein produced by the immune system in order to bind extremely tightly and specifically to a foreign substance. The backbone structure of antibodies vary, but the highly specific Y-shaped ends are an identifying feature;
Of these naturally occurring antibodies, IgG is the most used in synthetic biology with its simple and adaptable 4-chain structure;
Variable Heavy Chain - At the end of the constant heavy chain. Changes to bind to new target antigens.
Variable Light Chain - External, light chain that can bend around the variable heavy chain to better bind to the antigen.
Constant Heavy Chain - Base of the internal, long chain. Remains constant within all IgG of a particular species. The overall heavy chain structure also defines the antibody type.
Constant Light Chain - External light chain that binds the constant heavy chain, providing an elbow hinge for the bending of the variable light chain as it binds the antigen.
Antibodies are useful in the lab for identifying proteins of interest, during protocols such as ELISA or the Western Blot. Obtaining the specific antibodies necessary for a research project has inevitably meant paying fairly exorbitant fees to commercial producers. This is mostly due to the significant capital investment required to produce antibodies in the animal model. More recent developments in animal-free antibody production systems has brought down the price for the purchase of antibodies, but you may still wish to consider producing your own in house.
How are Antibodies made? (Animal Model)
The original models for scaled antibody production involve the exposure of a mammal (normally mice, but occasionally pigs, cattle, sheep, etc.) to the antigen of interest. The antigen is the region of a protein that an antibody binds to. The antigen may be provided on an intact protein, or as an isolated fragment of a whole protein. Regardless, the intention is to induce an immune response in the animal so that the bloodstream will contain a sufficient yield of antibodies. The types of antibodies yielded from such a process fit into two broad categories;
Monoclonal - Identical antibodies produced from a single cell line
Hybridoma cell lines are created by inoculating a mouse with an antigen, then removing B-lymphocyte cells from its spleen. These cells are fused with an immortal myeloma cell line and the resulting product screened for therapeutic efficiency.
Humanised mice are mice that have been genetically modified to contain the Human Constant + Heavy Light chains, allowing for a compatible human therapeutic product to be made in mice.
Creates a highly reproducible product in large volumes.
Specific to a single epitope (region within the antigen).
More expensive and time-consuming (> 6 months) to produce than polyclonal antibodies.
Polyclonal - Varied antibodies produced from different cell lines, all intent on producing a product that binds the same target antigen
Polyclonal antibodies are created by inoculating a lab animal (generally a mammal) with an antigen for several weeks in order to build up a large amount of antibodies in the bloodstream. The antiserum is extracted from the bloodstream, which will contain varied antibodies specific to the antigen provided.
The varied antibodies will bind to different epitopes within the antigen, allowing for greater accumulation around each protein. This makes polyclonal antibodies the better choice for protein detection in the lab. The addition of a fluorescent signal is also less likely to affect the functionality of all the antibodies in the group.
Each round of production will produce a different set of antibodies. Without the isolation from the larger organism, this process for producing polyclonal antibodies will never produce a perfectly consistent product.
Less expensive and time-consuming to produce than monoclonal antibodies (4-8 weeks)
The ethically questionable nature of these protocols is plain as day, fortunately better alternatives now exist. Those alternatives mostly revolve around our good old trusty single-celled expression systems.
Antibody Synthesis in Single-Celled Organisms
This is possible thanks to our understanding of how the protein sequence of an antibody known to bind a specific target protein can be reverse engineered into a DNA sequence for production in a biosynthetic strain. However using these systems still relies on foreknowledge of antibody sequences that can bind a target. Our digital tools are still unable to exhibit the uncanny accuracy of an immune system in rapidly prototyping antibody structures, so an animal may still need to be exposed with a pathogen at some stage. This is better than a production line of sickly animals being endlessly milked of their antiserum, but it is still not ideal.
Given time and investment in AI and algorithmic protein binding tools we may eliminate the need for wet-lab antibody discovery. This would effectively end the need for lab-animals in antibody science, as the synthesis can be moved into the far more efficient bacterial or yeast expression systems. As new and improved hosts for antibody synthesis are discovered or engineered, the endemic economies of scale will see these systems become the new norm.
Protocols:
I am working on unearthing some juicy protocols from an expert I know :)
This paper is great: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3906537/